LARYNGEAL PARALYSIS - POLYNEUROPATHY (LPN1, LPN2, & LPPN3)
Laryngeal Paralysis - Polyneuropathy (LPN1, LPN2, & LPPN3)

Leonberger dogs may suffer from neuromuscular disease collectively termed Laryngeal Paralysis - Polyneuropathy (LPPN). Many LPPN affected dogs suffers from laryngeal paralysis; clinical signs include noisy breathing, a change in their bark, or even difficulty breathing due to involvement of the larynx and laryngeal folds in the throat. Additionally, dogs may show signs of slowly worsening exercise intolerance and develop gait abnormalities, such as an exaggerated hitched step, especially in the hind limbs; there is often wasting of the hind limb muscles as well. Eventually the disease may progress to the point where surgical intervention for laryngeal paralysis is required or the dog cannot support its own weight. Biopsies of nerves from affected dogs show degradation of the nerve fibers and loss of myelin, the insulating material that normally helps speed messages along nerves. Muscle biopsies show atrophy resulting from nerve loss.
Dogs with LPPN may show only signs of laryngeal paralysis or only gait abnormalities at the onset of disease.
Research carried out at the University of Minnesota, the University of Bern, and the University of California, San Diego - Comparative Neuromuscular Laboratory indicates that polyneuropathy / laryngeal paralysis within the Leonberger breed is a group of several genetically distinct, but clinically similar diseases. We have mapped three major genetic risk loci and identified the causative mutations that we now term LPN1, LPN2, and LPPN3.
LEUKOENCEPHALOMYELOPATHY (LEMP)
Leukoencephalomyelopathy (LEMP)

The neurological disorder leukoencephalomyelopathy (LEMP) is a recessively inherited neurodegenerative disorder that affects the white matter of the central nervous system (CNS). Canine LEMP is characterized by slowly worsening gait abnormalities, especially spontaneous knuckling, dragging of the paws and hypermetria of the thoracic limbs, and a characteristic pattern on magnetic resonance imaging (MRI). Affected dogs show corresponding gross lesions in the cervical spinal cord white matter that may extend to the thoracic spinal cord, as well as to the brain; peripheral nerve and muscle biopsies are unremarkable. Canine LEMP often shows a juvenile onset and is characterized by a generalized progressive ataxia. Spinal reflexes of affected dogs are mostly normal. In the progressive clinical course of the disease, affected dogs may become increasingly immobile within a few months. Like many diseases of the CNS, there is no effective treatment for LEMP. Since in most cases the dog is not in pain, but is strongly restricted in its quality of life, owners are encouraged to ask a veterinarian for advice.
Research carried out at the University of Minnesota, the University of Bern, and Utrecht University has identified two LEMP mutations within the gene NAPEPLD, one in the Leonberger and the other in Rottweilers. The Rottweiler mutation has also been observed in Great Danes.
For more information about LEMP in Rottweilers and Great Danes click here.
LPPN3 - CNTNAP1
LPPN3 - CNTNAP1
LPPN3 is a form of laryngeal paralysis and polyneuropathy results from amino acid change within the gene CNTNAP1. It is inherited in an autosomal recessive manner, meaning that two copies of the mutation are required to show signs of disease (LPPN3-D/D). LPPN3 affected Leonberger dogs typically develop severe laryngeal paralysis at a young age, with most affected dogs requiring laryngeal tieback surgery. Additional clinical signs, which were noted variably among the dogs, included difficulty swallowing, changes in barking frequency and quality, high-stepping and uncoordinated gait, stumbling and tripping, exercise intolerance, and muscle atrophy. Like many neurological diseases, there is no effective treatment for LPN. Since in most cases the dog is not in pain but is strongly restricted in its quality of life, especially due to the frequent loss of normal function of the larynx, owners are encouraged to ask a veterinarian for advice.
- This mutation explains ~4% of all diagnosed cases of laryngeal paralysis-polyneuropathy in Leonbergers (average onset 3.5 years); population testing of >2,700 Leonbergers indicates that the carrier rate is ~11%.
- This mutation explains ~40% of young onset laryngeal paralysis-polyneuropathy within the Saint Bernard (average onset 2 years); population testing of >300 Saint Bernards indicates that the carrier rate is ~20%.
- This mutation has also been found to be a rare cause of laryngeal paralysis-polyneuropathy in Labrador retrievers (especially younger Labradors, average onset 7.5 years); population testing of >1,500 Labrador retrievers indicates that the carrier rate is ~10%.
- We have also identified LPPN3-D/D affected mixed breed dogs, and the mutation is present in several breeds.
LPN1 - ARHGEF10
LPN1 - ARHGEF10
LPN1 is a polyneuropathy resulting from a 10 base pair deletion within the gene ARHGEF10; dogs homozygous (LPN1-D/D) for the LPN1 mutation typically develop clinical signs of disease (including severe laryngeal paralysis) before they reach 3 years of age.
At present, LPN1-D/D dogs represent ~8% of all diagnosed cases of Leonberger polyneuropathy; a further ~23% of dogs in our research population with a phenotype consistent with or diagnosis of unexplained polyneuropathy have the LPN1-D/N genotype (compared to ~11% of healthy control dogs). The average age that clinical signs are first noted in these LPN1-D/N dogs, if they develop at all, is 6 years. Due to other causes of neuropathy in Leonbergers, the exact mode of inheritance of the LPN1 form of neuropathy cannot yet be stated for certain.
We have also identified LPN1 homozygous affected Saint Bernards with a biopsy confirmed polyneuropathy and clinical signs consistent with the disease. The LPN1 test can also be used in the Saint Bernard breed to aid in the diagnosis of polyneuropathy and to identify carriers in the breeding population.
- Population testing of >7,000 Leonbergers indicates that the carrier/at risk rate of this mutation is ~12%.
- Population testing of >350 Saint Bernards indicates that the carrier rate of this mutation is ~2%
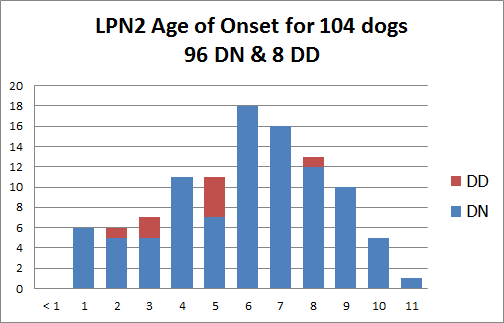
LPN2 - GJA9
LPN2 - GJA9
LPN2 is a partially penetrant autosomal dominant polyneuropathy resulting from a 2 base pair deletion within the gene GJA9; dogs heterozygous (D/N) and homozygous (D/D) for the LPN2 mutation may begin to show signs of disease as young as age 1, but may not show signs of disease until later in life or never at all. The average age of onset is 6 years.
The identified LPN2 mutation appears to be responsible for approximately 20-25% of the cases of polyneuropathy in Leonbergers.
- Population testing of >7,000 Leonbergers indicates that ~6% are LPN2 affected/susceptible.
LEMP - NAPEPLD
LEMP - NAPEPLD
LEMP in Leonbergers is a partially penetrant autosomal recessive central nervous system disease resulting from an amino acid change within the gene NAPEPLD. All Leonbergers with confirmed LEMP have tested homozygous affected (D/D) for this LEMP mutation; however, not all dog that are homozygous for this mutation may show obvious clinical signs of disease within their lifetime. Clinical signs may develop as early as 1 year of age.
- Population testing of >7,000 Leonbergers indicates that the carrier rate of this mutation is ~15%.
Funding
This work is generously supported by the Leonberger Health Foundation International, the Schweizerischer (Swiss) Leonberger Club, and the Deutscher Club Für Leonberger Hunde e.V. (German Leonberger Club), as well as from the proceeds of LPN & LEMP genetic testing.
Scientific References
A CNTNAP1 Missense Variant Is Associated with Canine Laryngeal Paralysis and Polyneuropathy.
Letko A, Minor KM, Friedenberg SG, et al. (2020)
Genes 2020, 11, 1426. doi: 10.3390/genes11121426
Genomic diversity and population structure of the Leonberger dog breed.
Letko A, Minor KM, Jagannathan V, et al. Genet Sel Evol 52, 61 (2020). doi: 10.1186/s12711-020-00581-3
Canine NAPEPLD-associated models of human myelin disorders.
Minor KM, Letko A, Becker D, et al. (2018)
Scientific Reports volume 8, Article number: 5818 (2018) doi: 10.1038/s41598-018-23938-7
A GJA9 frameshift variant is associated with polyneuropathy in Leonberger dogs.
Becker D, Minor KM, Letko A, et al. (2017)
BMC Genomics (2017) 18:662. doi: 10.1186/s12864-017-4081-z
An ARHGEF10 deletion is highly associated with a juvenile-onset inherited polyneuropathy in Leonberger and Saint Bernard dogs.
Ekenstedt KJ, Becker D, Minor KM, Shelton GD, Patterson EE, Bley T, et al. (2014)
PLoS Genet 10(10): e1004635. doi: 10.1371/journal.pgen.1004635
A novel leukoencephalomyelopathy of Leonberger dogs.
Oevermann A, Bley T, Konar M, Lang J, and Vandevelde M. (2008)
Journal of Veterinary Internal Medicine, 22: 467–471. doi: 10.1111/j.1939-1676.2008.0068.x
Inherited polyneuropathy in leonberger dogs: A mixed or intermediate form of charcot-marie-tooth disease?
Shelton GD, Podell M, Poncelet L, Schatzberg S, Patterson E, et al. (2003)
Muscle Nerve 27: 471–477. doi: 10.1002/mus.10350 doi: 10.1002/mus.10350